Latest Updates

Throne’s Stem Cell Educator Therapy is designed to reverse the root causes of autoimmune diseases, such as diabetes and alopecia, by fundamentally “resetting” the immune system through a one-time dialysis-like treatment using CB-SC stem cells.
Stem Cell Educator Therapy is recognized as the leading “Practical Cure Projects” for type 1 diabetes among 590 global technologies (JDCA State of the Cure 2021).
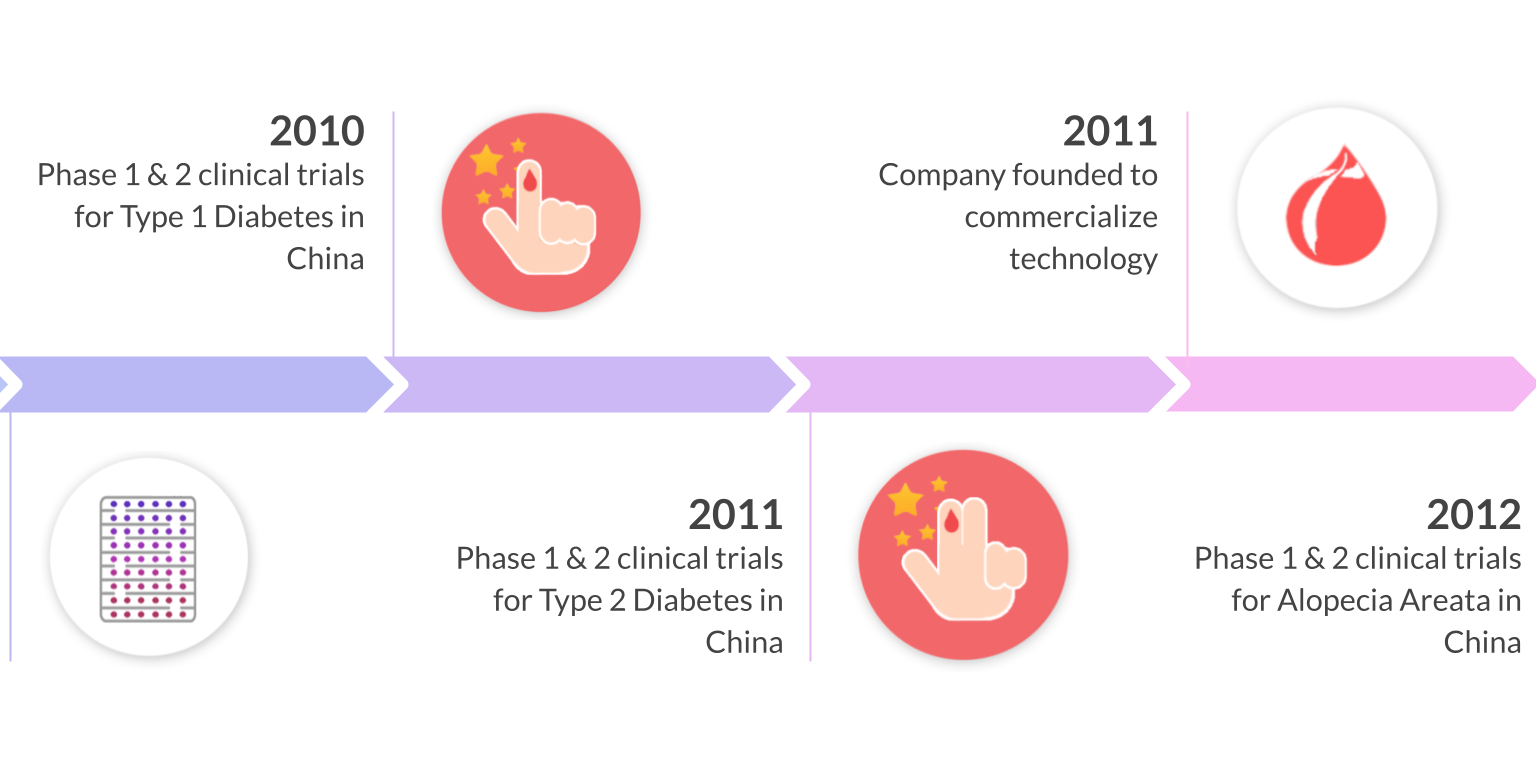
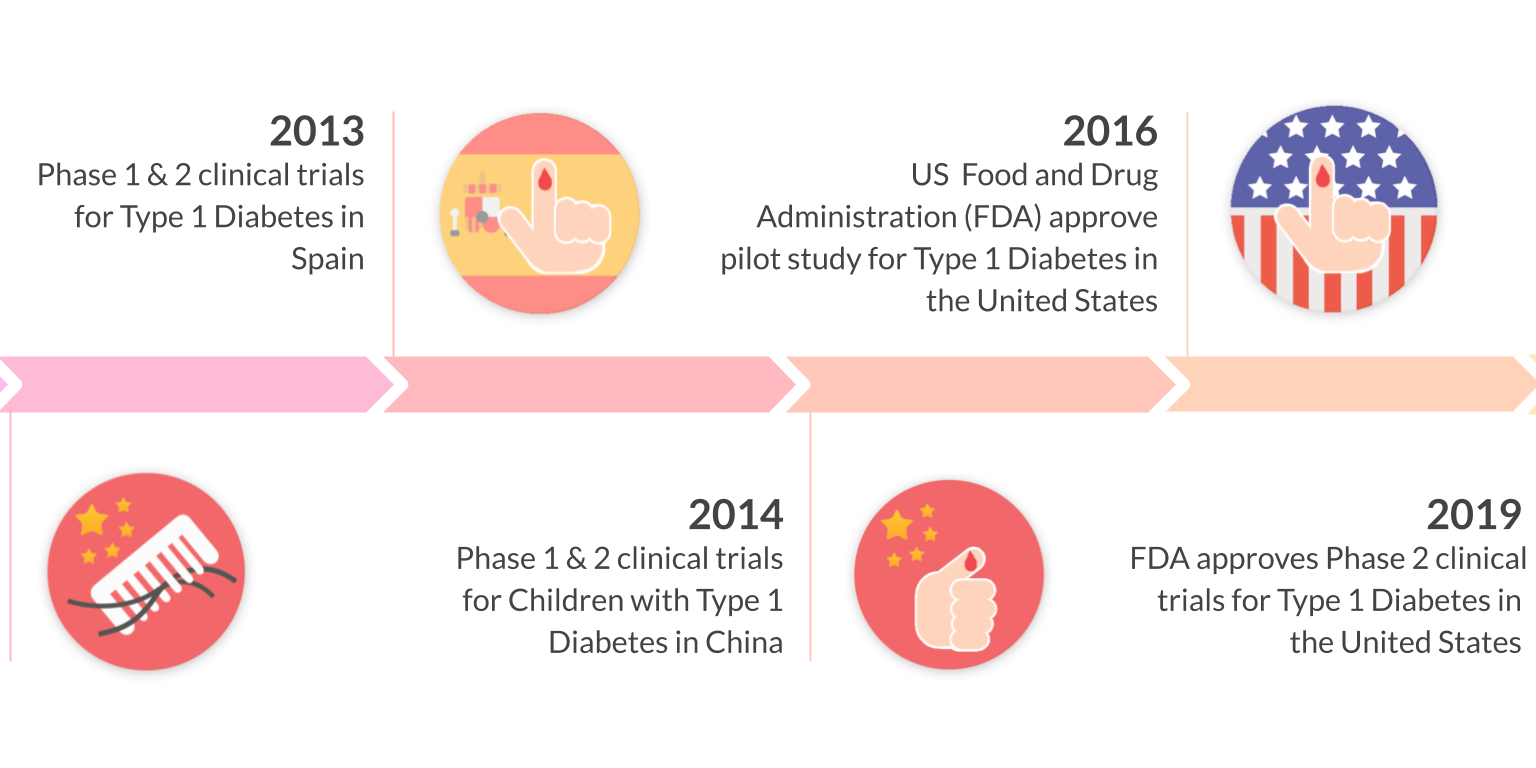
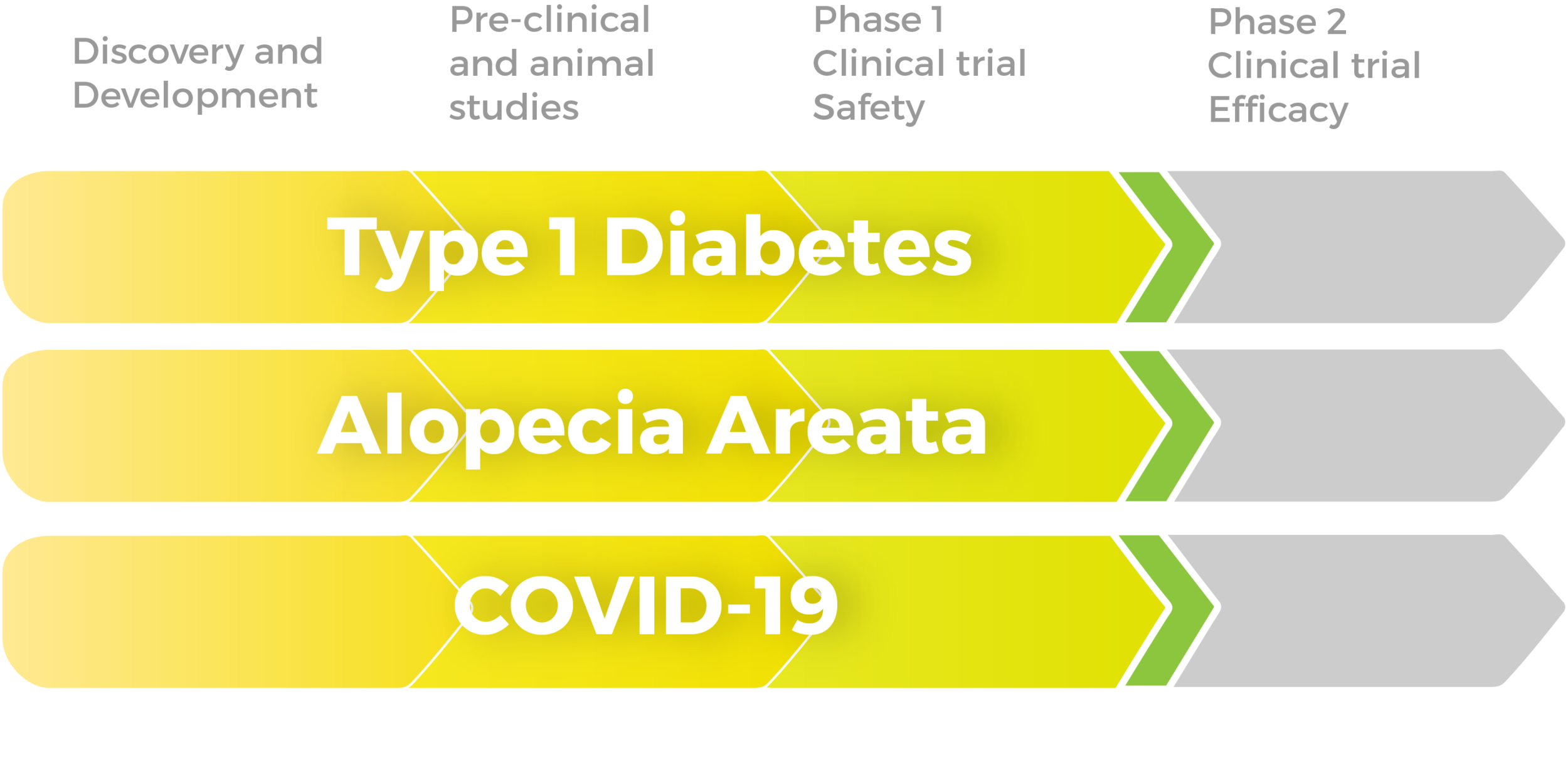
10 years of clinical studies in Spain, China, and United States have demonstrated the clinical safety and efficacy of Stem Cell Educator Therapy in over 200 patients ages 3-70. Throne is on track to commercializing this ready-to-use technology through three FDA phase 2 approvals for the treatment of type 1 diabetes, alopecia areata, and COVID-19.
It all started with a profound discovery:
The CB-SC Stem Cell
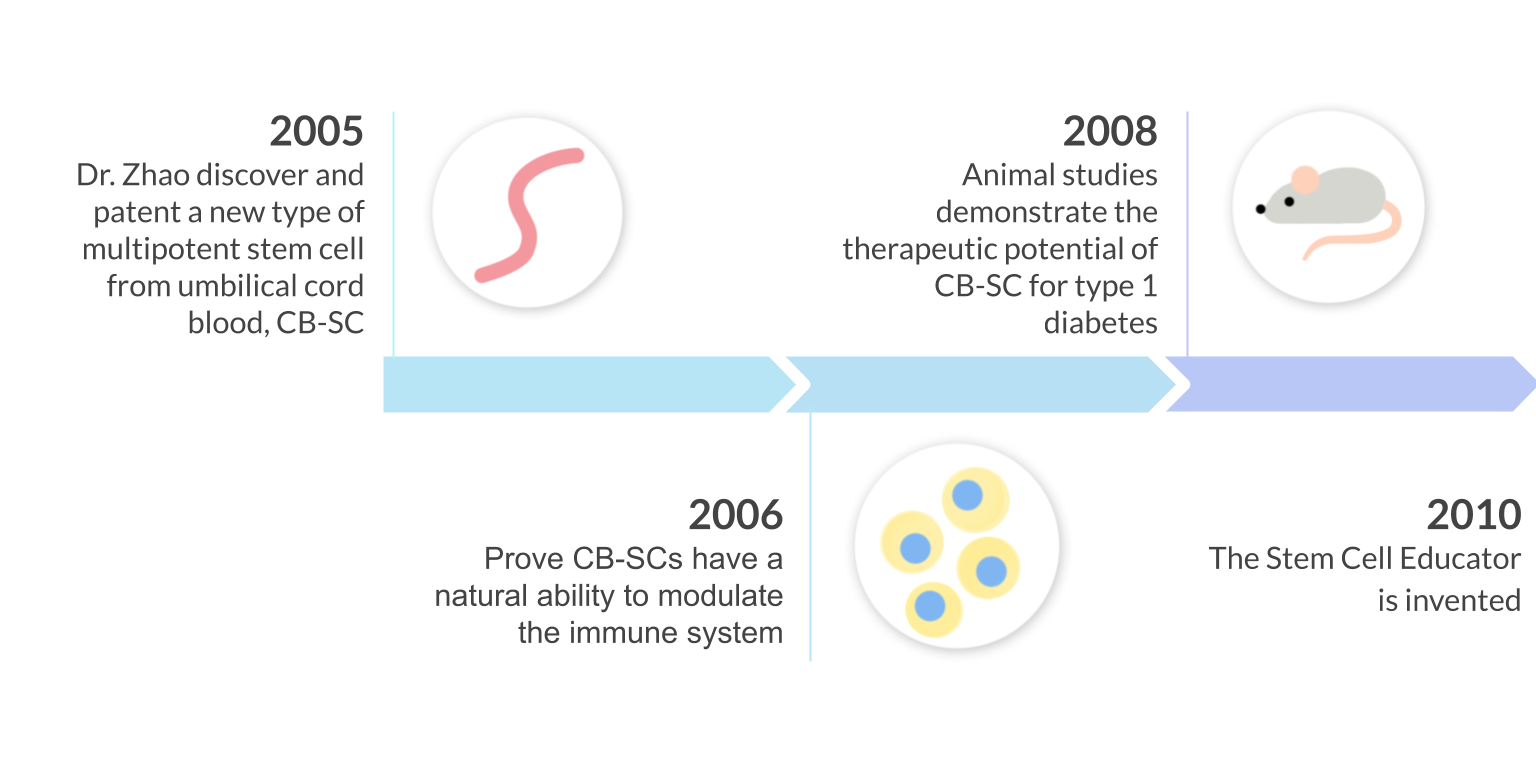
In 2005, Dr. Yong Zhao discovered and patented a new type of stem cell from the human umbilical-cord blood called CB-SC. These ethically derived stem cells are more powerful than any other previously discovered multi-potent stem cells due to their “immune educating abilities”. CB-SCs have the capability to restore autoimmunity and completely eliminate the requirement for stem cell genetic matching because of their low immunogenicity.
Using this new discovery, the Stem Cell Educator was invented to harness the immune modulating power of the CB-SCs for clinical application.
Stem Cell Educator Therapy is the only technology in the world that is licensed to use this stem cell. Educator Therapy does NOT use other stem cells like embryonic stem cells, iPSCs, MSCs, or HSCs.
CB-SC Stem Cells

Stem Cell Educator Therapy
The Immune System Reset
Stem Cell Educator Therapy (or “Educator Therapy” for short) is an ex-vivo process where a patient’s white blood cells are circulated through a Stem Cell Educator device coated with the umbilical cord-blood stem cells, CB-SCs. As this circulation occurs, the patient’s white blood cells intercept the CB-SC’s communications and are re-educated back to their pre-diseased state. This autologous process then circulates only the ‘educated’ white blood cells back into the patient (the CB-SCs never leave the Stem Cell Educator device). The newly ‘educated’ cells will now educate other defective cells in the patient’s body on who to fight and who to protect. This “immune reset” is designed to be minimally invasive, have virtually no side effects, and sustain long-term results.
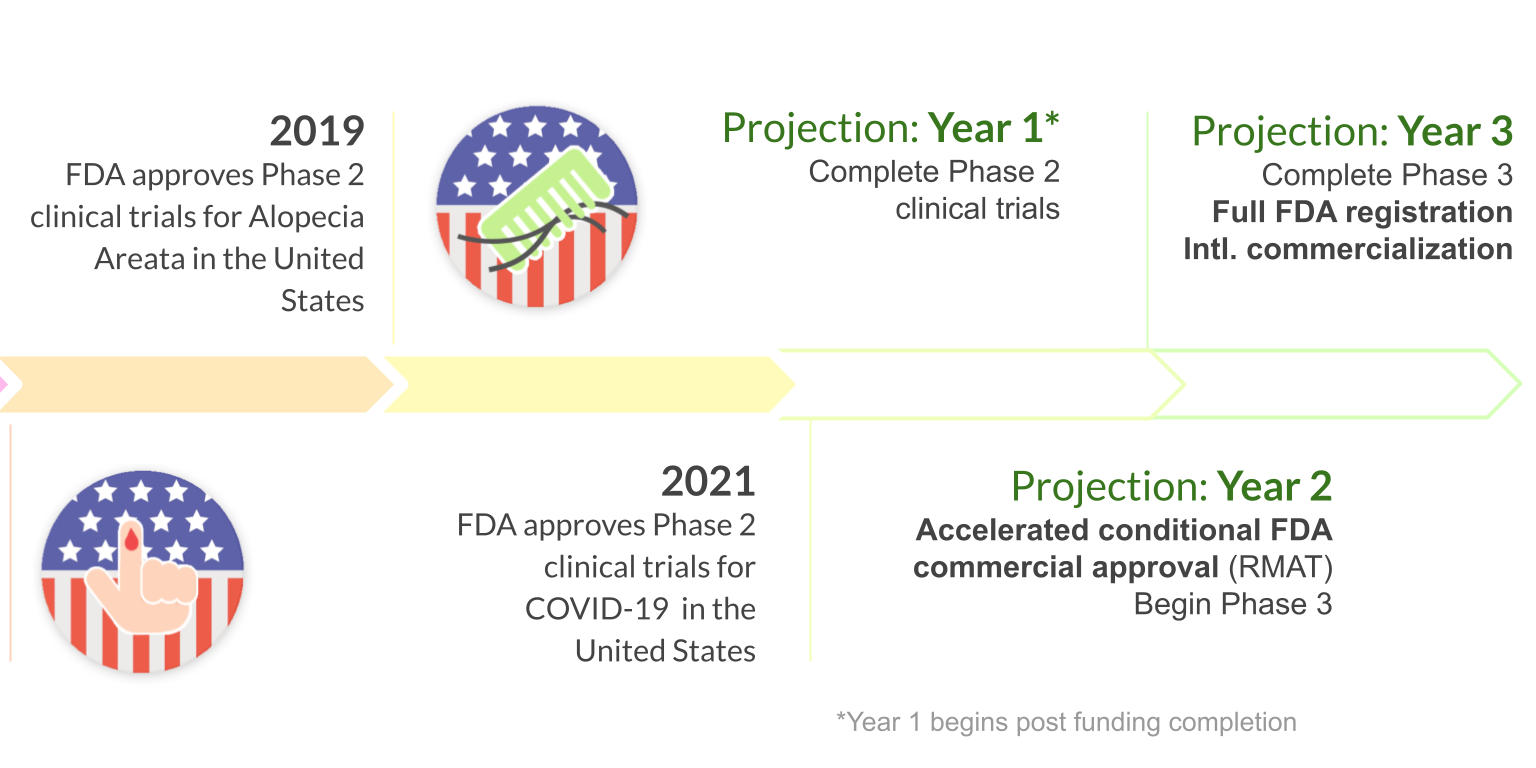
Throne aims to commercially launch its Stem Cell Educator Therapy once it receives regulatory approval.
Diabetes (type 1 and type 2)
Insulin injections are not a cure. They only treat the surface symptoms of diabetes. Stem Cell Educator Therapy aims to fundamentally correct the autoimmune destruction of islet beta cells and improve the beta cell function.
Clinical trials reveal that a single treatment of Educator Therapy can provide a lasting reversal of autoimmunity that allows regeneration of islet beta cells and improvement of metabolic control in people with type 1 (T1D) and type 2 (T2D) diabetes. Even long standing participants with over 20 years of type 1 diabetes have demonstrated increased C-peptides, improved sugar control, and regenerated beta cells post treatment. Similar clinical data were achieved in long-standing (> 25 years) severe type 2 diabetic patients, with markedly improving islet beta-cell function after receiving one treatment with Educator therapy.
Multi-center clinical trials in the United States, China, and Spain have demonstrated the safety and efficacy of Educator Therapy in more than 200 participants (including T1D and T2D) spanning from 3 - 70 years old. In follow up studies, more than 70% of participants who underwent Educator Therapy showed significant, statistical improvement in their immune markers. This improvement was demonstrated by measuring Tregs, Th1/Th2 cytokines, and autoimmune memory T-cells. Notably, insulin dosages have been reduced or stopped in some patients.
Type 1 Diabetes - 2.5 year follow up: minimal insulin dose and normal sugar control (a1c 5.2)
Type 1 Diabetes - Spain
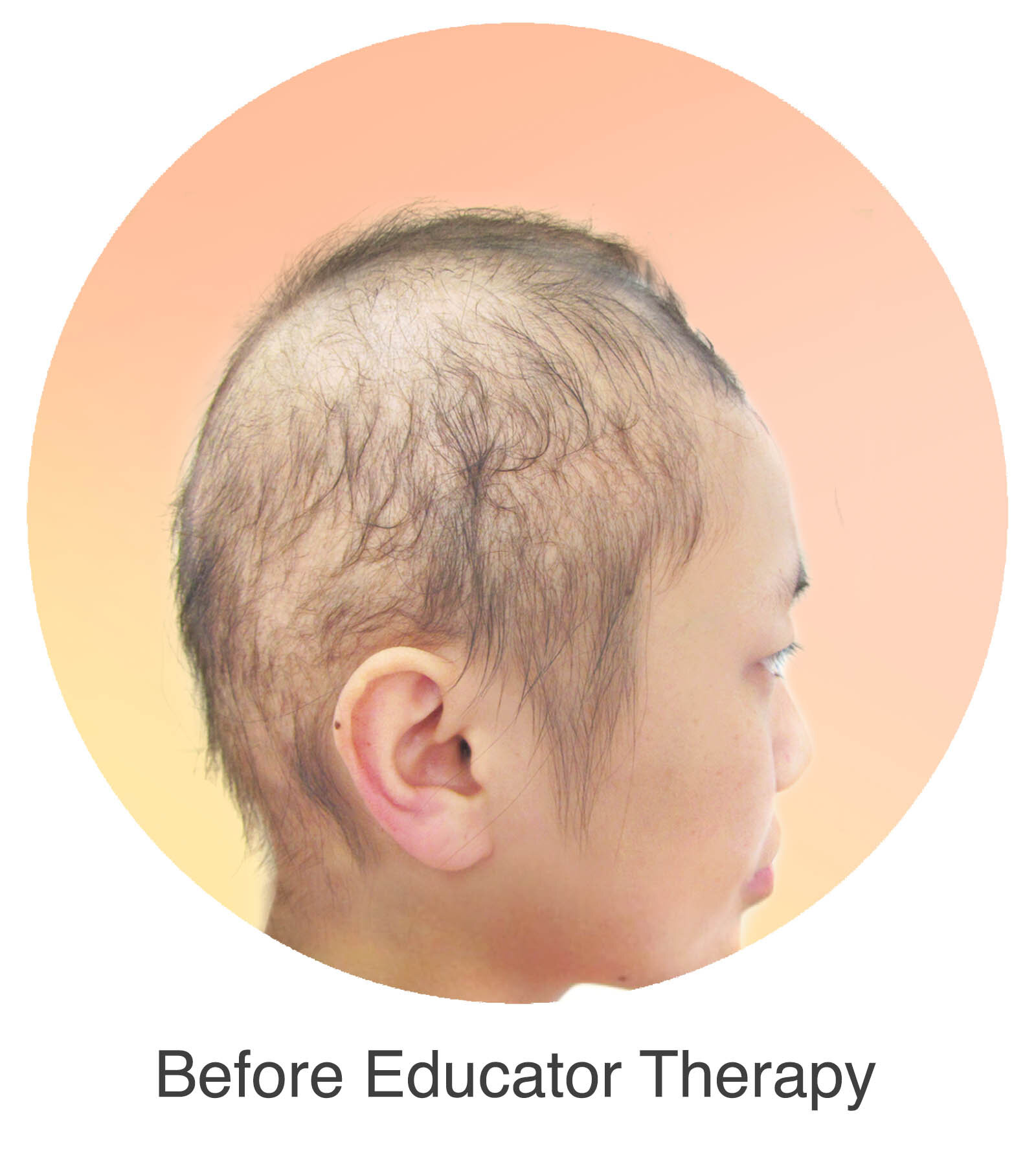
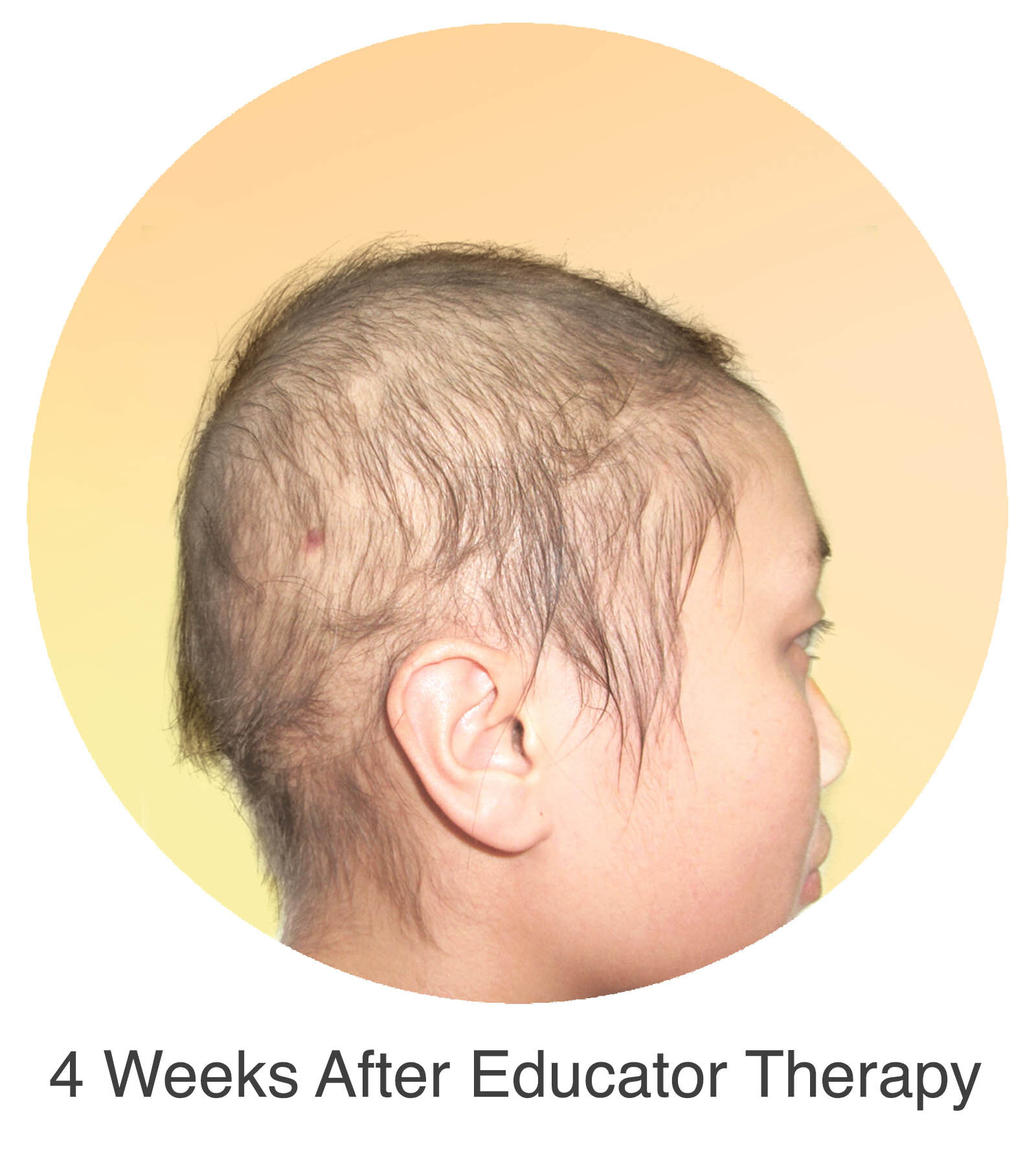
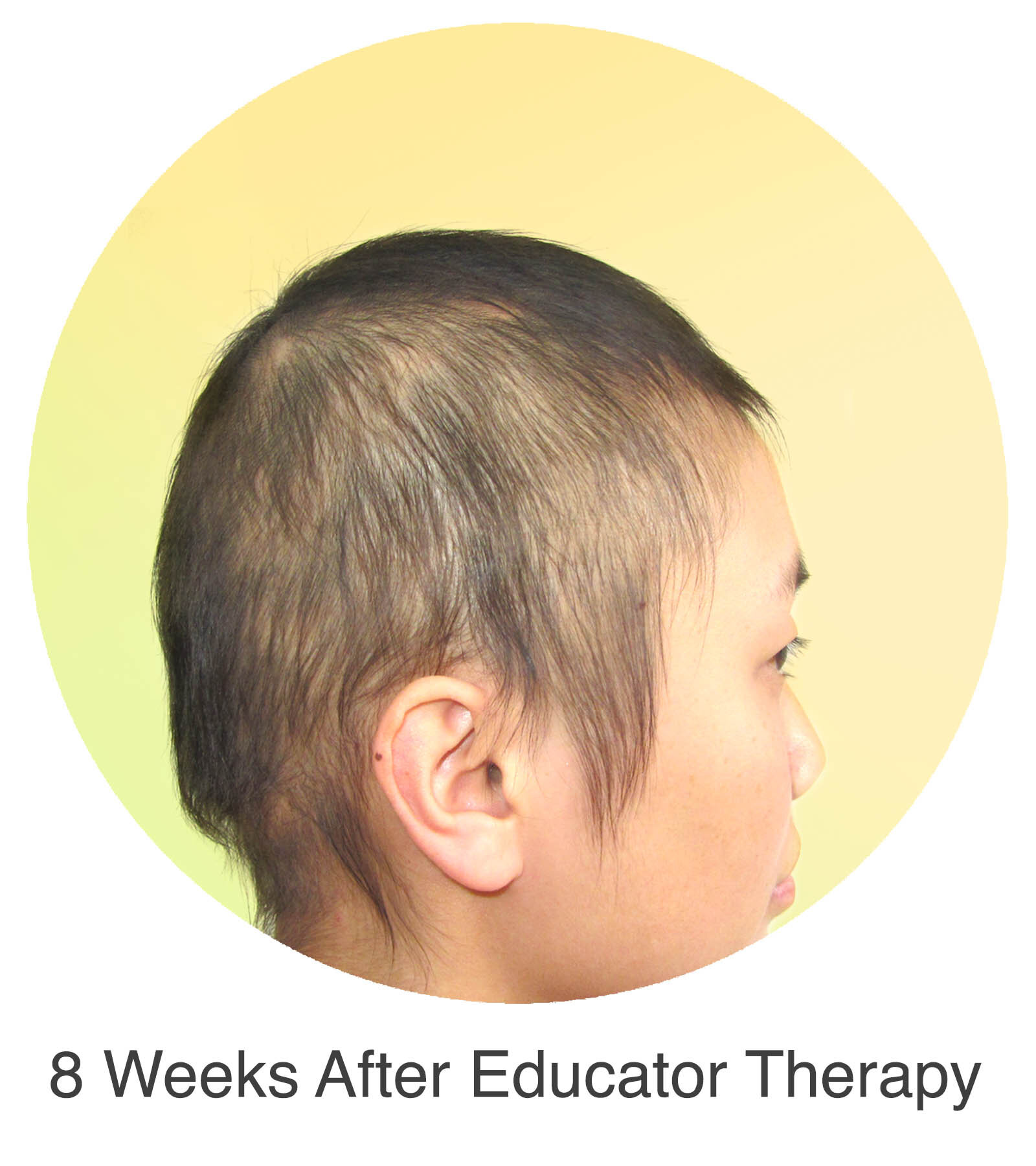
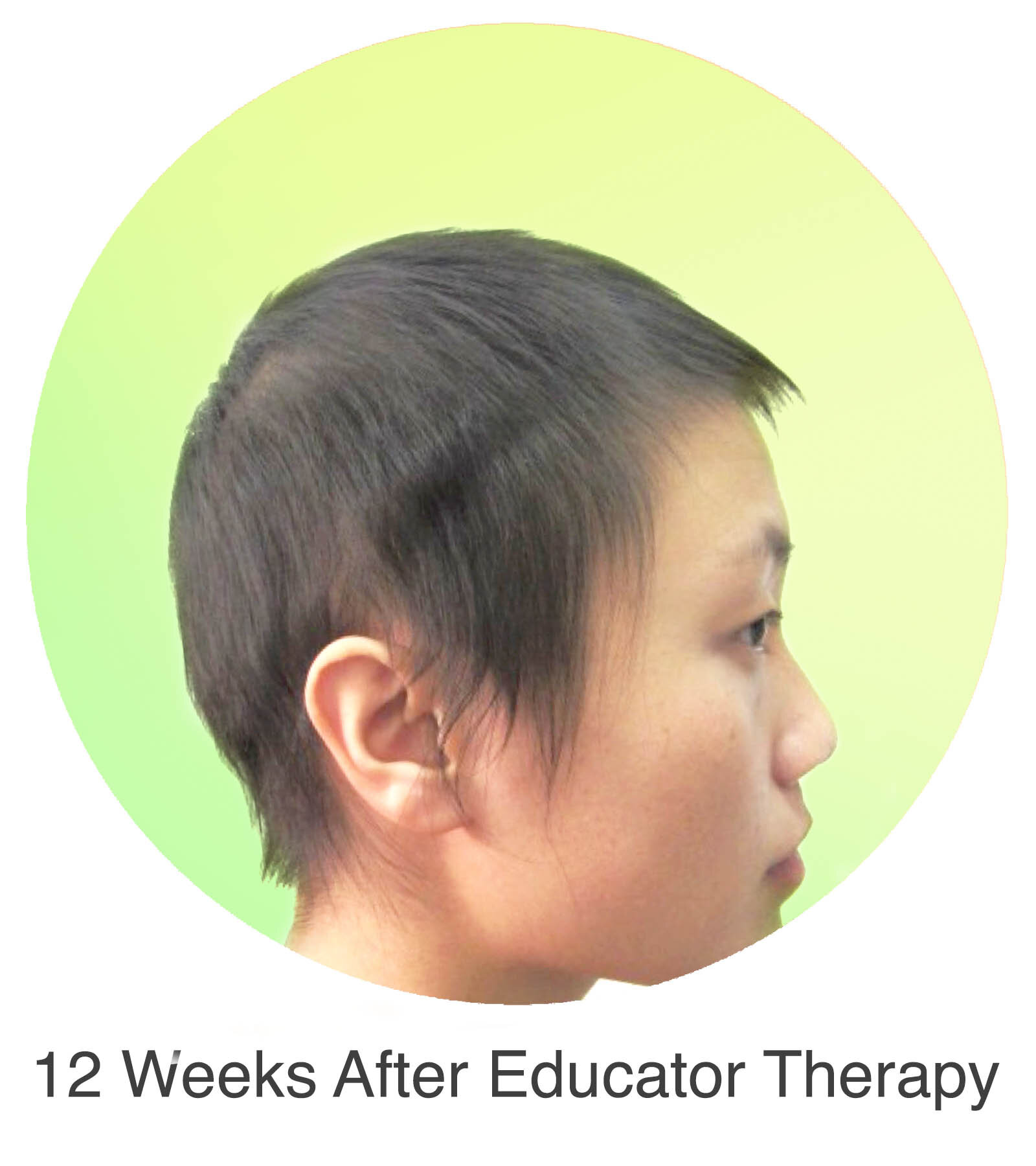
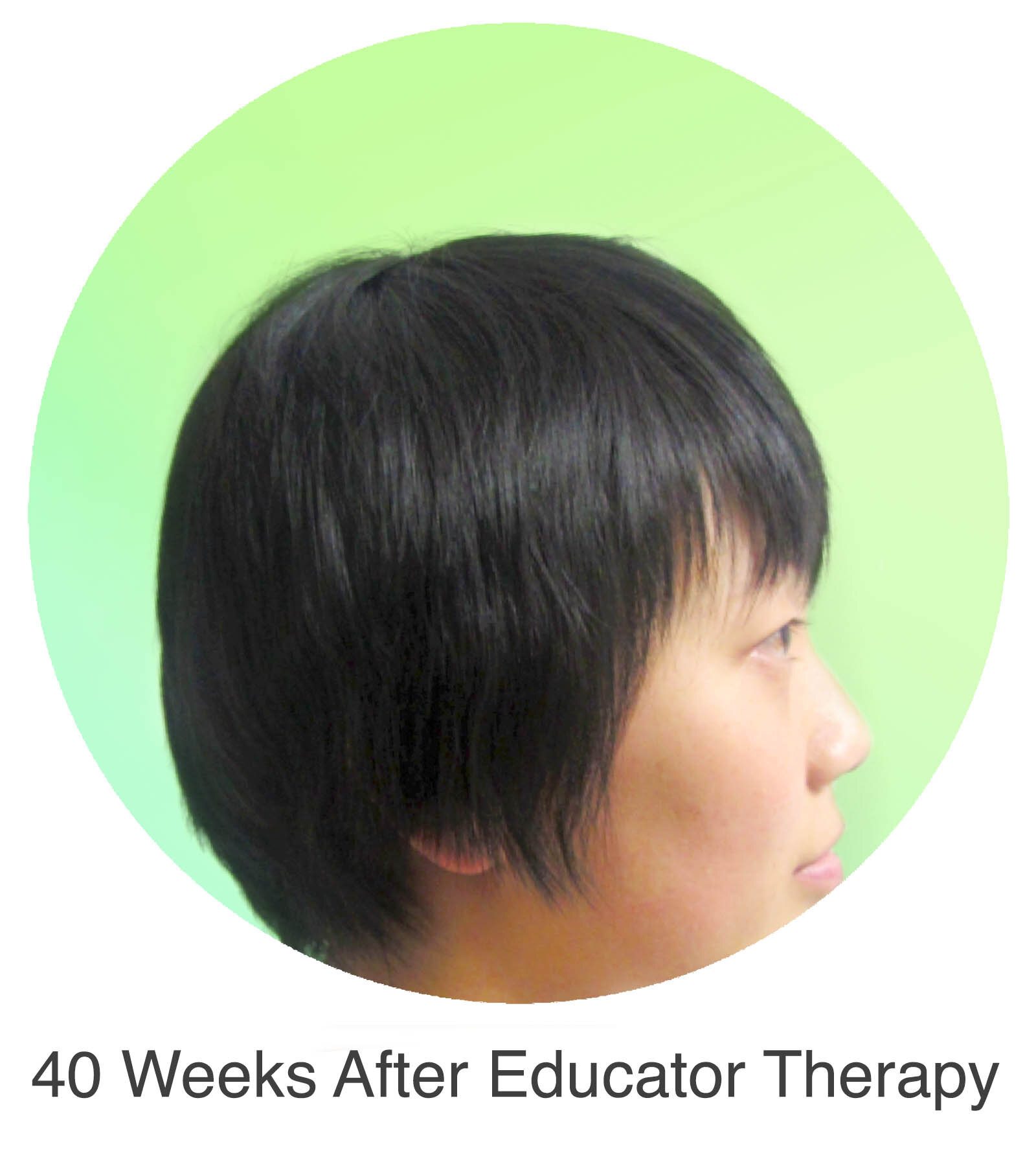
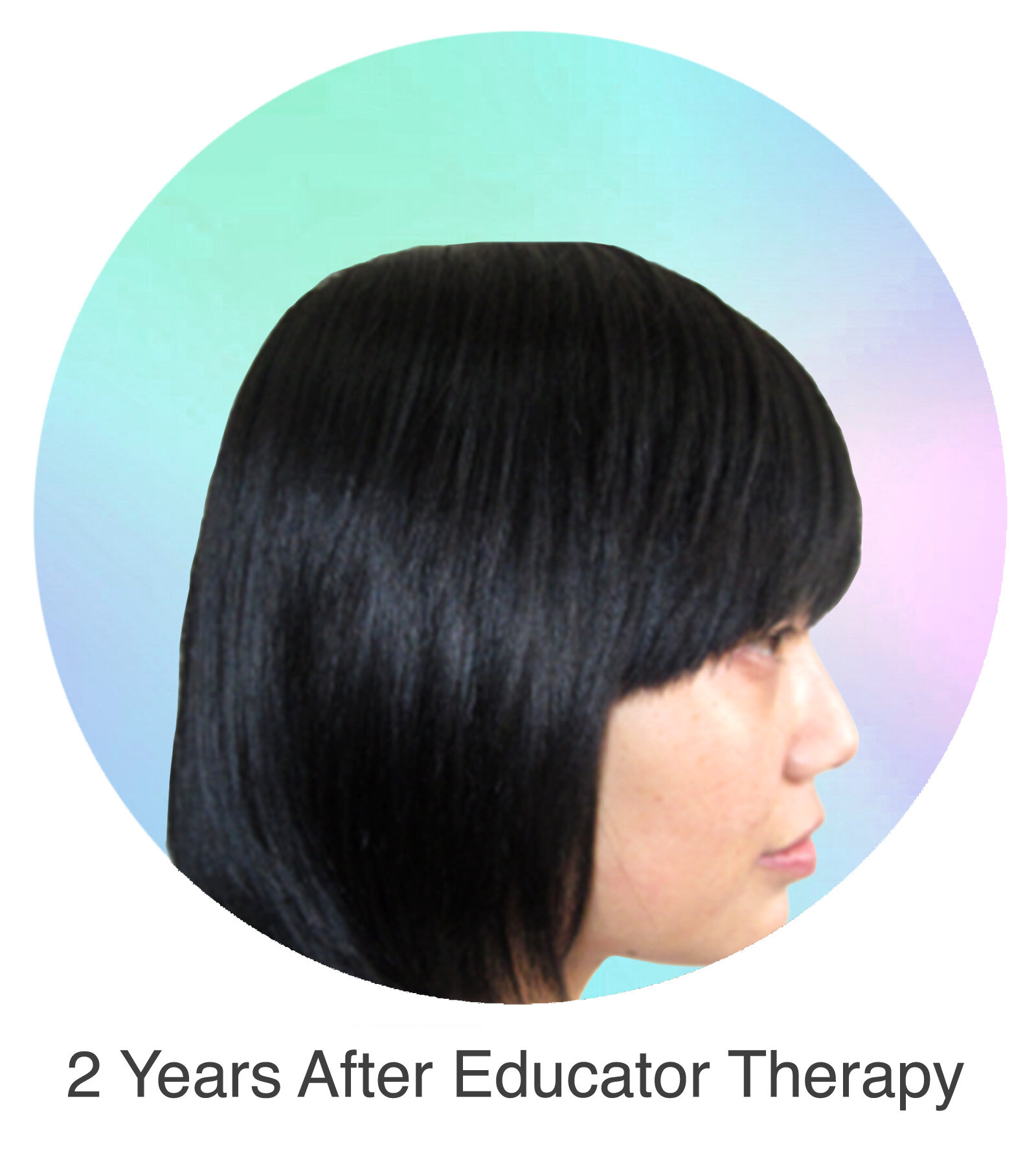
Alopecia Areata
Alopecia Areata is a common autoimmune disease that results in loss of body hair in varying degrees. Current immune-suppressant treatments such as JAK inhibitors can only temporarily subdue the effects of alopecia areata. A cessation of the medication will result in a 100% relapse.
Clinical studies have demonstrated Stem Cell Educator Therapy’s ability to establish systemic immune modulation and produce significant and/or complete hair regrowth by restoring the immune privilege of hair follicles. Additionally, Educator Therapy modifies rather than destroys the cells responsible for autoimmune responses, without increasing the chances of infection and tumor formation as demonstrated by long-term follow-up studies (four years post treatment). Severe patients with alopecia areata have demonstrated significant, sustained hair regrowth within 3-6 months of treatment.
COVID-19
COVID-19 has proved fatal to countless people with a compromised immune system. Similar to the principle mechanisms in treating autoimmune diseases, Stem Cell Educator Therapy aims to reset and strengthen the compromised immune systems and thereby reducing the high mortality rate among severe COVID-19 patients.
The infection of SARS-CoV-2 virus behind the COVID-19 pandemic involves multiple immune cells including T cells, B cells, monocytes, and macrophages. Conveniently, Stem Cell Educator Therapy has demonstrated its ability to correct multiple immune dysfunctions among these immune cells. In light of this, the new clinical trial will prove very effective in correcting the systemic inflammatory responses and their functional defects among COVID-19 patients.
Diseases Tested
Educator Therapy has been tested in the United States, China and Spain only on limited types of autoimmune diseases so far. The clinical data of these and other pilot trials have successfully demonstrated the principle mechanism of Stem Cell Educator Therapy—Immune Education. We aim to expand our research to other autoimmune diseases and beyond.

Research and Journal Publication
Over 20 years of research and more than 30 peer reviewed journal publications document the ongoing development process and other scientific findings relating to Stem Cell Educator Therapy. The publications include the linear progression of research ranging from the discovery of the CB-SCs, animal studies, clinical trials, to new findings.
Unrivaled Innovation
No stem cell injections
Stem Cell Educator Therapy utilizes an ex-vivo method, ensuring immune acceptance and eliminating risks of rejection. Stem cells are permanently contained in the Stem Cell Educator Device, ensuring no foreign cells enter the patient’s body.
No stem cells from animals or aborted fetuses
Many stem cell therapies use stem cells from animals and aborted fetuses which can result in safety risks, ethical and religious concerns. Stem Cell Educator Therapy eliminates these issues and thereby opens many markets traditionally closed to stem cell therapies.
Safety
Stem Cell Educator Therapy is a minimally invasive treatment that takes place outside of one’s body. Clinical trials demonstrate this therapy has minimal to negligible side effects.
Proven Research
Over 15 years of research and more than 200 clinical trial subjects from around the world demonstrate the safety and efficacy of Stem Cell Educator Therapy.
World's only CB-SC stem cell technology
Stem Cell Educator Therapy is the only technology in the world that uses the CB-SC which is more powerful than any other stem cell in its immune-modulating abilities. They have the capability to correct autoimmunity, restore immune balance, and eliminate the requirement for stem cell genetic matching.
Unlimited expansion of technology
Stem Cell Educator Therapy has unlimited application potential. Clinical evidence already demonstrates its efficacy for diabetes, alopecia, and other autoimmune diseases. Continued research aims to demonstrate its efficacy in other rare and incurable diseases.
Progress
How we got here and where we are going
FDA Pipeline

“Results from a groundbreaking study...indicate that stem cells derived from human umbilical cords may help overcome the autoimmune response that causes type 1 diabetes.”
“The treatment [Stem Cell Educator Therapy]...even worked in people with long-standing diabetes who were believed to have no insulin-producing ability.”
“One year later, the patients who got the treatment continue to manufacture some of their own insulin and eight have reduced their insulin shots by about 38 per cent.”