Stem Cell Educator Therapy
Introducing: The CB-SC Stem Cell
Stem Cell Educator Therapy would not be possible without CB-SCs: human umbilical cord blood-derived multipotent stem cells. Unlike all other stem cells including hematopoietic, mesenchymal, endothelial progenitor and monocyte-derived stem cells [4], CB-SCs are unique for a number of distinct reasons. First, they are negative for blood cell lineage markers while they display embryonic cell markers. Second, CB-SCs have very low immunogenicity and eliminate the need for genetic stem cell matching [5]. Third, they have the capability to attach tightly to culture dishes with a large rounded morphology and are resistant to common detaching methods (trypsin/EDTA). This distinct characteristic allows for a seamless process of collecting suspended lymphocytes that have been treated by CB-SCs without the risk of mixing them after ex-vivo co-culture [6]. Stem Cell Educator therapy affords every patient the assurance that only their own “educated” lymphocytes will return to their body during the treatment.
Stem Cell Educator Therapy
Dr. Yong Zhao developed Stem Cell Educator Therapy [7] based on his successful results in non-obese diabetic (NOD) mice and other pre-clinical evidence evidence supporting the capability of CB-SCs to control autoimmune responses by altering Tregs and human islet b cell-specific T cell clones.
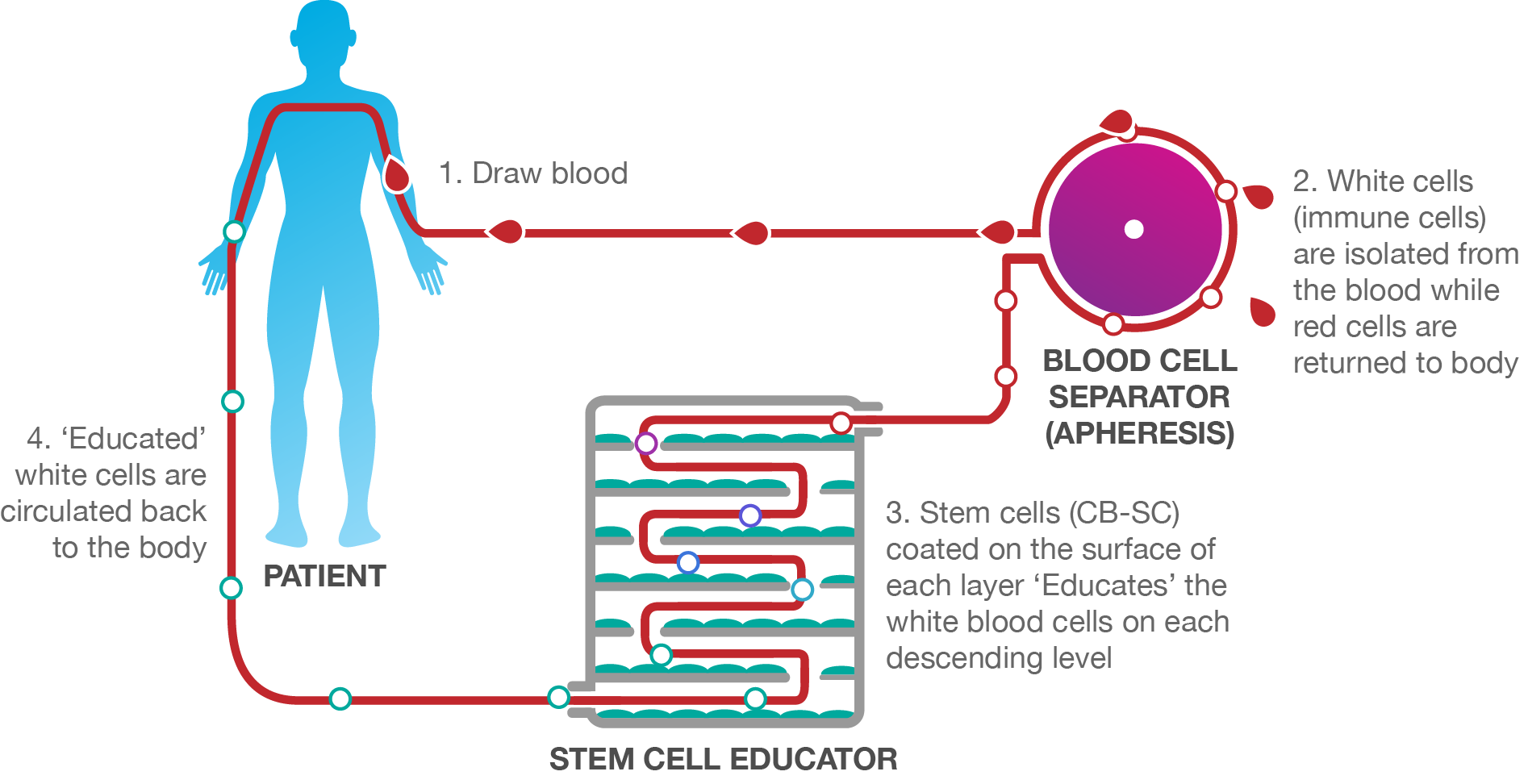
Stem Cell Educator Therapy (i.e. Educator Therapy) is an in vitro process where a patient’s white blood cells are circulated through a Stem Cell Educator device that is coated with human umbilical cord blood-derived multipotent stem cells, CB-SCs. As this circulation occurs, the patient’s white blood cells (MNCs) intercept the CB-SC’s communications, re-educating the cells back to their pre-diseased state. This process then circulates only the ‘educated’ white blood cells back into the patient (the CB-SCs never leave the Stem Cell Educator device). The newly ‘educated’ cells then educate other defective cells in the patient’s body on who to fight and who to protect. This immune reset allows the patient’s immune system to fight for its healing the way it was initially designed.
Unlike other stem cell treatments, Educator Therapy does not introduce stem cells or reagents into patients, ensuring 100% immune acceptance and zero risk of rejection.
What about safety and efficacy?
Our published and unpublished data [7] has demonstrated Educator Therapy to be entirely safe and non-invasive. Unlike any other stem cell treatment, Educator Therapy does not introduce stem cells or reagents into patients, ensuring 100% immune acceptance and zero risk of rejection.
Individuals who undergo Educator Therapy experience minimal to zero side effects. Minimal side effects include slight discomfort from the apheresis process (blood draw).
What Sets Educator Therapy Apart?
No stem cell injections, transplantations, or blood transfusions occur. Educator Therapy utilizes an in vitro method, ensuring immune acceptance and eliminating risks of rejection. Stem cells are permanently contained in the Educator Device, ensuring no foreign cells enter the patient’s body.
CB-SCs. Discovered and patented by Dr. Yong Zhao, Educator Therapy is the only technology in the world licensed to use these powerful multipotent stem cells. They are the only stem cell with the capability to restore autoimmunity and completely eliminate the requirement for stem cell genetic matching because of their low immunogenicity.
Zero stem cells come from animals or aborted fetuses. These stem cell treatment methods often result in safety risks, ethical and religious concerns. Educator Therapy is ethically responsible.
A Closer Look at Educator Therapy in Type 1 Diabetes
The immune dysfunction of type 1 diabetes (T1D) is complicated. There are several different tiers of the immune system (e.g. T cells, Tregs, B cells) contributing to the autoimmune responses. To address these different tiers, a comprehensive immune modulation approach is needed.
Findings from our clinical trials provide powerful evidence that a single treatment of Educator Therapy provides lasting reversal of autoimmunity, allowing the regeneration of islet b cells and improving the metabolic control in individuals with long-standing type 1 diabetes [7]. Additionally, individuals experienced markedly improved C-peptide levels, reduced median glycated hemoglobin A1C values (HbA1C) and decreased daily insulin doses. Notably, a single treatment could improve islet b function that lasts a year.
In an open-label, Phase 1 and Phase 2 study, 15 individuals with T1D received one treatment of Educator Therapy. Their median age was 29 years (range, 15 to 41) and their median diabetic history was 8 years (range, 1 to 21). The results of these studies revealed increased expressions of costimulating molecules (specifically CD28 and ICOS), increased numbers of Tregs and CD4, CD25 and Foxp3, as well as restored Th1/Th2/Th3 cytokine balances.
These trial findings indicate that Educator Therapy with CB-SCs meets the scientific expectation that a successful T1D therapy will have the capability to address different arms of the autoimmune response and restore balance to the immune system through systemic and local modulations. This is a capability that Educator Therapy has clearly demonstrated through our clinical data and animal studies [1, 7, 8, 12].
Currently, we are performing an international multi-center Phase 2 clinical trial to improve the efficacy of Educator Therapy. For more information, please visit ClinicalTrial.gov.
Clinical Case Report—Type 1 Diabetes
Case 1: A T1D patient (female, 15-year old, 5-year diabetic duration) received one treatment of Educator Therapy, with one-year follow-up. C-peptide response following a 75-g oral glucose tolerance test (OGTT) reveals a significant improvement of b-cell function by Educator Therapy. The dashed red line indicates the lower limit for normal C-peptide levels in Chinese populations. To convert C-peptide value to nmol/L, multiply the ng/ml by 0.331.
Case 2: C-peptide response following a 75-g OGTT from another severe T1D subject (male, 40-year old, 17-year diabetic duration) received one treatment with Stem Cell Educator therapy, with one-year follow-up. The level of 0.01 ng/ml is the minimum detectable level (sensitivity, the dashed purple line) of C-peptide by radioimmunoassay (RIA). To convert C-peptide value to nmol/L, multiply the ng/ml by 0.331.
Educator Therapy in Type 2 Diabetes
A successful approach to Type 2 Diabetes (T2D) is one that will have the ability to overcome immune dysfunction, as this will be a key tool in addressing the immune response of insulin resistance in T2D patients.
In an open label, Phase 1 and Phase 2 study, patients (N = 36) with long-standing T2D were divided into three groups (Group A, oral medications, N = 18; Group B, oral medications + insulin injections, N = 11; Group C having impaired b-cell function with oral medications + insulin injections, N = 7). All patients received one treatment of Educator Therapy.
The results of these studies revealed improved metabolic control and reduced inflammation markers. Notably, homeostasis model assessment (HOMA) of insulin resistance (HOMA-IR) demonstrated that insulin sensitivity was improved post treatment (Figure Left). Islet-beta cell functions in Group C were markedly recovered (Figure Right) and C-peptide levels were restored.
Mechanistic studies as well as clinical data from current Phase 1 and Phase 2 studies have revealed that Educator Therapy reverses immune dysfunctions through immune modulation on monocytes, balances Th1/Th2/Th3 cytokine production, and produces lasting improvement in metabolic control for individuals with moderate or severe T2D who receive a single treatment. In addition, Educator Therapy does not have the safety and ethical concerns associated with conventional stem cell-based approaches.
Clinical Case Report—Type 2 Diabetes
Case 1: Before receiving Educator Therapy, patient A (male, 52 years old, 12 years diabetic duration) had been treated with current medications (e.g., metformin, sulfonylureas, pioglitazone) but was unable to control his blood sugar levels (retaining at ~10 mmol/L), despite his insulin dosage increasing to 70 U/day. He had been hospitalized with a minor stroke (left brain). This patient displayed the typical insulin resistance, as demonstrated by a 75-g OGTT test with normal levels of fasting and stimulated C-peptide. After receiving Educator Therapy, his insulin dosage decreased by 20 U/day one month later. At 6 months post treatment, his insulin dosage had continually decreased to 50%, with occurrence of hypoglycemia if injected a little bit more insulin. Notably, his HbA1C level was reduced from base line 9.4% to 7.1% by month 6 (note: 7% is the ADA-recommending standard for diabetics). His fasting blood sugar levels retained at 6 ~ 7 mmol/L. This data demonstrates that Educator Therapy can markedly improve insulin sensitivity and overcome insulin resistance (the key issue of T2D).
Case 2: A long-standing diabetic patient B (female, 57 years old, 27 years diabetic duration, 32 U insulin/day) displayed an impaired islet beta cell function with the fasting C-peptide level 0.16 ng/ml (note: o.6 ng/ml is the lower limit for normal C-peptide levels in Chinese populations.). After receiving Educator Therapy for 3 months, her fasting C-peptide level markedly increased to 0.54 ng/ml; correspondingly and her daily insulin dosage decreased by 50%. Her HbA1C level was reduced from base line 7.3 % to 6.4% by month 3. This data indicates that Educator Therapy can restore the islet beta cell function and improve the metabolic control.